Bumedinst 6010.13 quality assurance qa program – The BUMEDINST 6010.13 Quality Assurance (QA) Program stands as a cornerstone in the medical equipment and device industry, ensuring the highest standards of quality and accuracy. This comprehensive program provides a systematic approach to monitoring, evaluating, and improving the quality of medical devices, safeguarding patient safety and enhancing healthcare outcomes.
By implementing rigorous inspection and testing procedures, collecting and analyzing data, and implementing corrective and preventive actions, the BUMEDINST 6010.13 QA Program empowers healthcare providers with the confidence that the medical equipment they rely on meets the most stringent quality standards.
Quality Assurance (QA) Program Overview: Bumedinst 6010.13 Quality Assurance Qa Program
The QA program for “bumedinst 6010.13” defines the purpose and scope of quality assurance for medical equipment and devices. It Artikels key principles and methodologies used in the program, emphasizing the importance of QA in ensuring the quality and accuracy of these devices.
QA Program Implementation
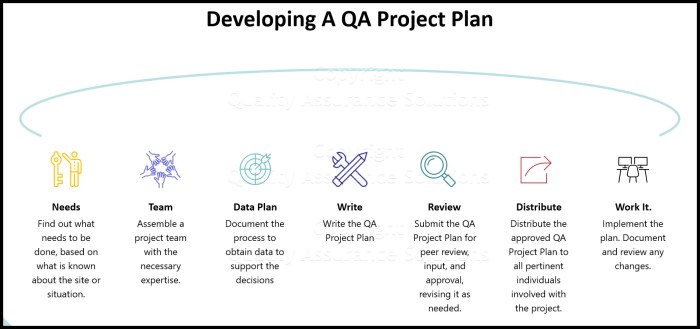
Implementing the QA program involves planning, resource allocation, and training. Stakeholders have defined roles and responsibilities in the QA process. Best practices for QA program implementation include:
- Establishing clear goals and objectives
- Identifying and allocating necessary resources
- Providing comprehensive training to all involved personnel
QA Program Components

The QA program encompasses various components:
- Inspection and testing procedures:Verifying device performance and compliance with specifications.
- Data collection and analysis:Gathering and evaluating data to identify trends and potential issues.
- Corrective and preventive action (CAPA) processes:Implementing measures to address and prevent quality issues.
- Quality improvement initiatives:Continuously enhancing the QA program and device quality.
QA Program Evaluation and Improvement
Evaluating the QA program’s effectiveness is crucial. Monitoring and assessment methods include:
- Performance audits
- Review of quality metrics
- Customer feedback
Evaluation results drive improvements, such as:
- Refining inspection and testing procedures
- Enhancing data collection and analysis techniques
- Implementing new CAPA processes
Case Studies and Examples
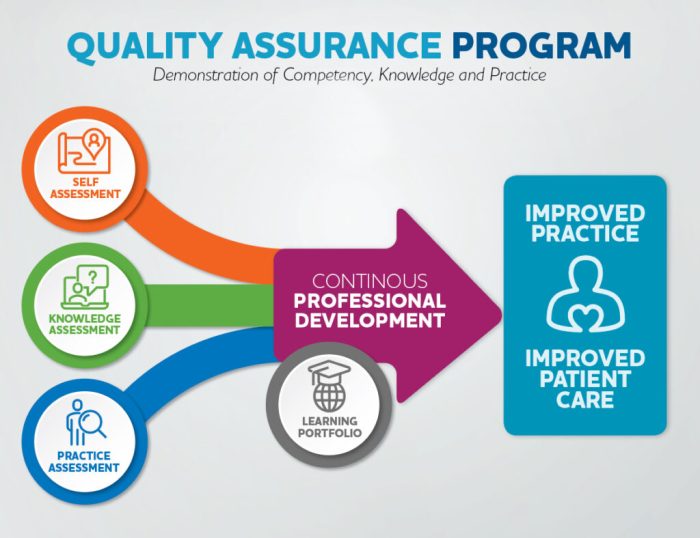
Successful QA programs in the medical equipment and device industry demonstrate their impact. Case studies highlight:
- Improved device quality and patient safety
- Reduced product recalls and customer complaints
- Enhanced compliance with regulatory standards
Challenges and lessons learned from these case studies provide valuable insights for QA program development.
Commonly Asked Questions
What is the purpose of the BUMEDINST 6010.13 QA Program?
The BUMEDINST 6010.13 QA Program aims to ensure the quality and accuracy of medical equipment and devices, safeguarding patient safety and enhancing healthcare outcomes.
What are the key principles of the BUMEDINST 6010.13 QA Program?
The BUMEDINST 6010.13 QA Program is guided by principles of continuous improvement, risk management, and data-driven decision-making.
Who is responsible for implementing the BUMEDINST 6010.13 QA Program?
Healthcare organizations are responsible for implementing and maintaining the BUMEDINST 6010.13 QA Program within their facilities.
How does the BUMEDINST 6010.13 QA Program contribute to patient safety?
By ensuring the quality and accuracy of medical equipment and devices, the BUMEDINST 6010.13 QA Program directly contributes to patient safety by reducing the risk of equipment-related incidents and adverse events.